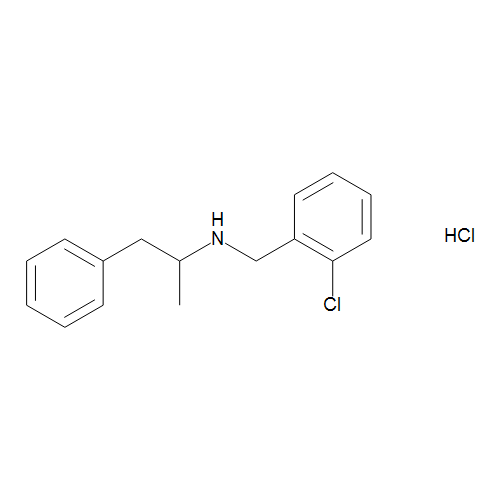
Clobenzorex is the chemical name of a central nervous system (CNS) psychostimulant, a prodrug of dextroamphetamine, and an anorectic (appetite suppressant) drug chemically classified as an N-substituted amphetamine, amphetamine derivative, and a phenethylamine. Marketed by Aventis as the branded product Asenlix, clobenzorex is also produced and distributed under generic trade names, including Dinintel, Finedal, Rexigen, and Itravil, all of which are widely accessible in pharmacies, available for purchase over the counter, and legal for personal use in Mexico as an appetite suppressant. A prodrug of dextroamphetamine, clobenzorex is metabolized within hours of ingestion into 4-hydroxyclobenzorex and, in smaller amounts, d-amphetamine.
Chemistry
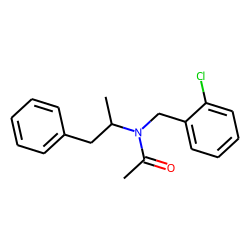
Synthesis
Condensation between amphetamine (1) and 2-chlorobenzaldehyde (2) gives a Schiff-base, CID:135056236 (3). Subsequent reduction with sodium borohydride completed the synthesis of clobenzorex (4).
Urinalysis
Clobenzorex can be detected by urine drug screening. It is one of many drugs that can cause a positive result for amphetamine in urine drug screening. It may be differentiated from use of amphetamine itself through testing for metabolites such as 4-hydroxyclobenzorex or enantiomeric analysis.
Legal Status
Brazil
In Brazil, clobenzorex is a controlled prohibited psychotropic (class A3).
Canada
In Canada, clobenzorex is not specifically listed per the Controlled Drugs and Substances Act.
Mexico
In Mexico, clobenzorex is available for sale over the counter under trade names including Asenlix and Itravil.
United Kingdom
In the United Kingdom, clobenzorex is a controlled drug (class B).
United States
Clobenzorex is not scheduled under the Controlled Substances Act of 1970 nor is it controlled under the Federal Analogue Act, despite being a derivative of benzphetamine. It is not subject to import controls and is legal to import and possess for personal use, provided the following conditions are met:
- it is used to treat a condition with no FDA-approved medications or an orphan drug does not effectively treat a condition, and the risks of use have not been determined to outweigh to benefits of treatment
- is not being deceptively and unlawfully marketed
- is part of an ongoing medical treatment plan that began in a foreign country
World Anti-Doping Agency
The use of clobenzorex is banned by the World Anti-Doping Agency for use during sports competitions as an athletic performance enhancer ("doping").
See also
- Amfepramone or "diethylpropion" (U.S. name), Tepanil is standard form; Tenuate Dospan extended-release, rarely prescribed
- Vyvanse, a prodrug of dextroamphetamine, to which it converts only after metabolism of the bonded lysine
- Mefenorex (Rondimen, Anexate, Pondinil), a prodrug of racemic amphetamine
- Phenylpropanolamine (PPA), a mild sympathomimetic nasal decongestant, discontinued in the U.S. circa 2000-2001
- Phenmetrazine, powerful amphetamine derivative and anorectic "diet pill" branded Preludin, discontinued in the U.S. circa 1982
- Phendimetrazine (Bontril PDM and TrimTabs), a prodrug of phenmetrazine and anorectic, discontinued in the U.S. circa 1997
- Propylhexedrine (Benzedrex brand inhaler sold over the counter (OTC) with stimulating effects
- Sympathomimetic, class of activating drugs, e.g. ephedrine, pseudoephedrine; fenproporex, PPA; often decongestants
References